Glowing materials have fascinated scientists for decades, inspiring technologies from bioimaging to OLED displays. While traditional explanations of fluorescence focus on specific molecular structures, new findings suggest that interactions within molecular aggregates may hold the key to a broader understanding of this phenomenon.
A collaboration between researchers in Trieste and Tel Aviv investigated this puzzle by combining simulations and experiments to study intrinsic fluorescence, focusing on the amino acid cysteine — a molecule not known to fluoresce on its own.
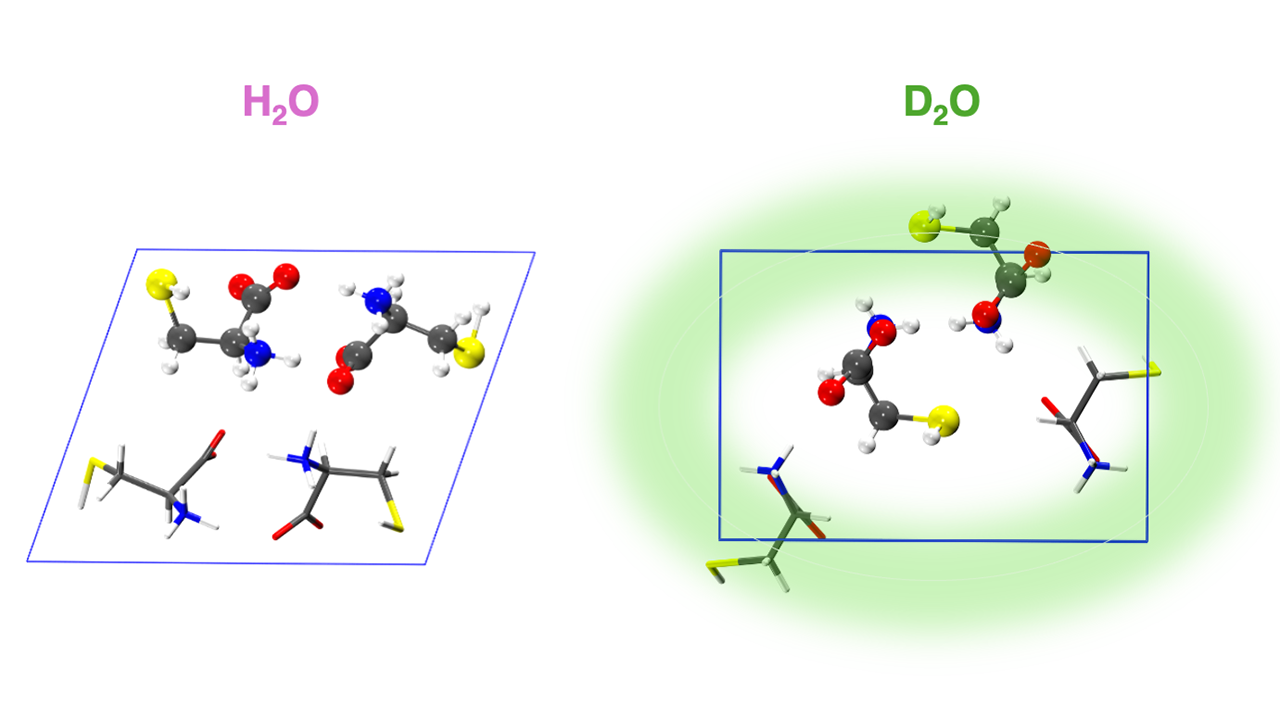
The study began with a striking observation by the group of Ehud Gazit at Tel Aviv University: cysteine crystals grown in light water (H₂O) and heavy water (D₂O) formed two different crystalline structures, despite having identical molecular constituents. These structural differences had a profound impact on their optical behavior — only one of the two forms was fluorescent. This discovery highlights how subtle changes in the crystallization environment can lead to divergent molecular packing and emergent optical properties.
“The beauty of this system is that we’re not dealing with complex or disordered aggregates,” explains Debarshi Banerjee, a PhD student at the International School for Advanced Studies (SISSA) and ICTP, and the study’s first author. “We’re working on single crystals of a non-fluorescent amino acid. This allows us to do an exact one-to-one comparison between the theoretical predictions and the experiments.”
To probe the mechanisms behind this difference, the team led by ICTP senior research scientist Ali Hassanali in Trieste performed state-of-the-art molecular simulations that mirrored the experimental setup. “Approximations that are usually valid in most first-principles molecular dynamics simulations do not hold when one wants to look at the dynamics of electronic excited states, which makes these simulations extremely challenging,” Banerjee explains.
The simulations revealed that in the fluorescent crystal, vibrational modes were more constrained, giving the system more time to emit visible photons. In contrast, the non-fluorescent crystal was more flexible, leading to rapid internal conversion without the emission of a photon.
These findings expand our understanding of the ground-principles behind fluorescence, a topic that is currently being actively pursued by the ICTP-led ERC funded project HyBOP (Hydrogen-Bond Networks as Optical Probes), paving the way for new experiments in the field. The research also opens up new exciting questions on why heavy water (D₂O) can have such a big impact on crystal nucleation.
Read the full article.