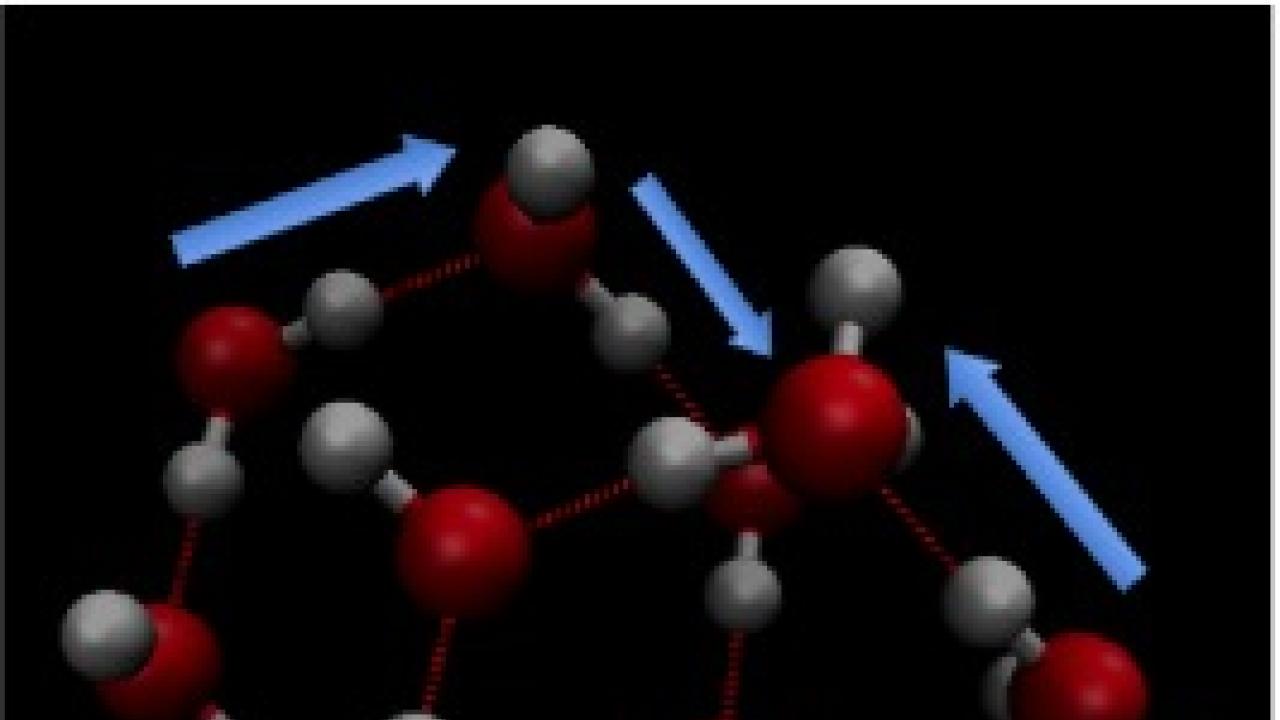
Water owes many of its unique properties to intermolecular interactions, particularly hydrogen bonds, that physics models have not yet fully described. Roberto Car, a professor of chemistry at Princeton University, is studying the quantum nature of the protons involved in those bonds in hopes of better explaining water's behaviour.
"It's a seemingly simple molecule, but when they get together they give rise to all sorts of fascinating structures," said Car in a seminar held at ICTP on 26 July.
Though several models of water exist, they treat the molecule as a classical particle, which doesn't account for many experimental observations of water's behaviour. For example, Car said that for protons in both liquid water and ice, "the momentum distribution differs considerably from the classical equilibrium," which creates very large effects on the system, both structurally and dynamically.
Recent studies in Car's research group have found evidence that the protons involved in hydrogen bonds may undergo quantum tunnelling, where they cross an energy barrier higher than the proton's energy level. Tunnelling is a phenomenon specific to quantum mechanics. In ice under high pressure, this tunnelling is concerted in order to preserve local charge neutrality and avoid high-energy charge effects.
"The quantum nature of the proton is important and has to be taken into account," Car said. He added that, both in ambient conditions and at high pressure, the behaviour of water is "a real manifestation of quantum mechanics." The current research focuses on ice at conditions of low temperature and high pressure.
Car said this research was "curiosity driven," and he hopes it will answer basic questions about discrepancies between the expected and observed behaviour of water molecules.
In 2009, Car was awarded ICTP's Dirac Medal, along with collaborator Michele Parrinello, for their development of an ab initio simulation method that combines the quantitative electronic energy calculations of Density Functional Theory, with Newtonian simulations of atomic motions.
This Car-Parinello method, which is widely used throughout physics, chemistry, and material science, played a role in the new research Car presented at the seminar.