Water is liquid. Indeed, this is true at ambient conditions, as experienced in our daily life. But what would happen under extreme pressures and temperatures such as those inside planets rich in water like Uranus or Neptune? According to scientists, a new phase would appear, a form of water both liquid and solid: a “superionic” water. A team of researchers at ICTP and SISSA, among which Sandro Scandolo and Erio Tosatti, already theoretically predicted this almost 20 years ago in a study published in Science in 1999. Their paper was recently cited by a research team from Lawrence Livermore National Laboratory (LLNL), the University of California, Berkeley and the University of Rochester, which has provided the first experimental evidence for the existence of superionic water. A recent note about the study, featured in Nature Physics, was posted by the New York Times last week.
We have interviewed ICTP researcher Sandro Scandolo to find out more about the relevance and the impact of this new finding.
 |
What is superionic water?
Water is a simple molecule, two hydrogens attached to one oxygen. The three atoms normally form a V-shape. When squeezed, the Vs connect in an airy structure, the hydrogens and oxygens shuffle into other crystal structures forming one of the over 15 known crystalline structures of ice. Superionic water occurs at extreme conditions of pressure and temperature: the heat breaks the chemical bonds between atoms, the high pressure keeps oxygens in a fixed crystal structure while it forces positively charged hydrogen nuclei to flow freely within the lattice. This results in a phase which is both liquid and solid. This material is a conductor of electricity, like a metal, but the current is carried by positively charged ions.
Where can we find superionic water?
Superionic water does not exist naturally anywhere on Earth, but it may be abundant on other neighboring planets of the solar system, in the mantles of Uranus and Neptune for example. It’s also likely to be found in some of the exoplanets recently discovered. From an experimental point of view, back in 1999 the technologies available didn’t allow experimental physicists to recreate superionic water in their laboratories. Nevertheless, we did predict its existence through numerical simulations. It was a true challenge, especially considering the limited processing power of computers at the time.
 |
But now scientists have experimentally proved your initial prediction. How?
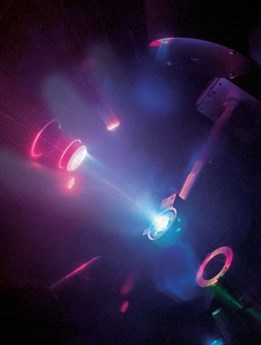
In the study recently published in Nature Physics, researchers have first squeezed water between two pieces of diamonds, reaching a pressure 25,000 times greater than the air pressing on our heads and obtaining one of the over 15 known types of ice, ice VII (60% denser than usual water). A pulse of laser light was then shot onto the sample of compressed ice. The subsequent shock wave, within 10 to 20 billionths of a second, has heated the sample up to 5,000 degrees and applied a 200 gigaPascal pressure (2 milllion times Earth’s atmosphere). Under these conditions, they got the first experimental proof of a superionic phase.
|
|
|
| Credit M. Millot/E. Kowaluk/J.Wickboldt/LLNL/LLE/NIF |
What is so remarkable about this study and what are future implications?
It’s the first time scientists manage to recreate such extreme conditions in a lab, the only conditions under which to observe superionic water. Most of the credit for this result goes to the incredible technological development of recent years and to the cutting-edge facilities exploited. The confirmation of the existence of this form of water validates the theoretical and computational tools used to predict it.
Thus, we can now use the same tools to engineer new materials or to study “extreme” physical situations we haven’t direct access to. We might even be able to answer questions that experts of other fields are asking. Let’s think about planets. We still know very little about them, but the exact phase of their components can become crucial to better investigate the thermal and mechanical properties of far-away planets.
---- Anna Lombardi