Metals might seem commonplace, explored and experimented with for a large variety of uses in daily life and beyond. But even typical simple metals can behave strangely under different conditions, with changes in the structure of their atoms adding up to give the metal unexpected properties. Two ICTP scientists have a new paper published in Nature Physics on how the atomic structure of a simple metal transforms under pressure, to become a brand-new type of liquid, that is somehow denser than a solid.
Victor Naden Robinson and Sandro Scandolo, a postdoctoral fellow and a staff scientist in ICTP's Condensed Matter and Statistical Physics section respectively, were building off of recent experimental discoveries of the strange behavior of metals under pressure. Separating the effects of crystalline structure from the effects of pressure, Robinson, Scandolo, and colleagues at Xi’an Jiaotong University and the University of Edinburgh modeled what happened to the atoms, ions, and electrons in liquid potassium, their example metal, when pressure increased.
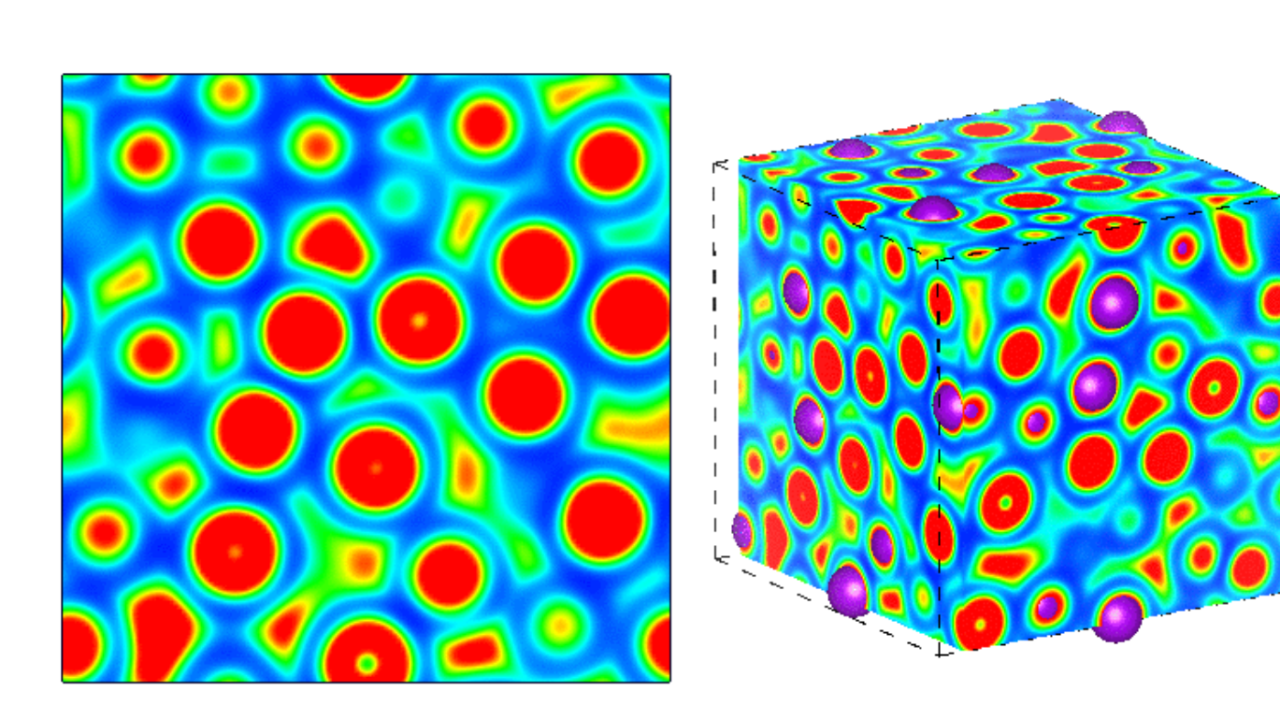
To Scandolo and Robinson, one of the most interesting strange changes of these metals under pressure is what happens to the electrons. With increasing pressure, the electrons are no longer zipping around in semi-spherical clouds around the cores of the atoms, delocalized. They instead congregate in the gaps between the atoms, clustering the electrons' negative charges to create something similar to an ion, a charged atom. But that cluster doesn't have a nucleus, so it's called a "pseudoanion." Scandolo explains: "The electrons are stripped away from the potassium atoms and pushed to fill the pockets between the atoms, or rather what remains of the atoms, now positively charged ions."
With these pseudoanions negatively charged, and the potassium nuclei positively charged, the material stays liquid but transforms from a metal into another material, known as an electride. "Solid-solid transitions are ubiquitous in nature, like the solid-solid transition in cocoa butter that determines the change of color of chocolate when refrigerated," says Scandolo. "But phase transitions between two liquids are extremely rare."
What makes that transition more interesting, says Scandolo, is that it's not abrupt and uniform, so that the macroscopic properties of the material can switch back and forth during the transition. Between a metal and an electride, properties like heat capacity, thermal expansion, the speed that sounds travels through the material, and reflectivity change as the underlying electronic structure changes.
That phase change is not the only interesting aspect of potassium becoming an electride under pressure: as an electride, the liquid becomes denser than the solid. "Surprisingly, we find that liquid potassium is denser than the "close-packed" solid from which it melts," says Scandolo. This shouldn't happen: atoms in liquids typically have more energy, so they move around more, taking up more space than atoms stable in a solid do.
"There is only a limited number of ways in which one can "pack" atoms in a liquid or in a solid," says Scandolo. Close-packing is one of those ways, like how grocers sometimes stack oranges in pyramids. "If you took a system of hard spheres, like oranges," says Robinson, "then close-packing is the most efficient packing of these spheres. For a system to be denser than this, there has to be some mechanism which allows the spheres to either distort or get smaller so that you can pack them more efficiently." Potassium being denser as a liquid than a solid challenges the notion of close-packing as the most efficient way to arrange atoms in space.
That mystery, of a liquid somehow denser than a solid, was solved as the group investigated the dynamics of the electron structure of liquid potassium. "The atoms are no longer to be assumed to be well approximated by spheres, due to their transformation into a more complex "electride" complexes, made of cations and pseudoanions," says Scandolo. Robinson continues: "With increasing pressure, the liquid becomes more electride-like with bonding which is still more or less ionic. But becoming electride-like also allows the system to compress the electrons much more."
"The most surprising part of this study for me," says Scandolo, "is the finding that a simple elemental metal like potassium, a textbook example of "normal" behavior when studied at ambient conditions, displays instead such a rich and interesting behavior when it is compressed to moderate pressures." For Robinson, "I have always thought that the liquid alkali metals would form electrides under pressure, so I am glad to see that we can quantify this."
"The excitement comes at the realization that the transformation does not originate simply from a better packing of the atoms," Scandolo says, "but from a dramatic change of the underlying electronic structure."
Zong, H., Robinson, V.N., Hermann, A. et al. Free electron to electride transition in dense liquid potassium. Nat. Phys. (2021). https://doi.org/10.1038/s41567-021-01244-w
---- Kelsey Calhoun